Technology & Innovation

Innovation Advancing the Future of Supply Chain Integrity
Smart systems, validated processes, and proprietary technologies to protect the most critical life sciences
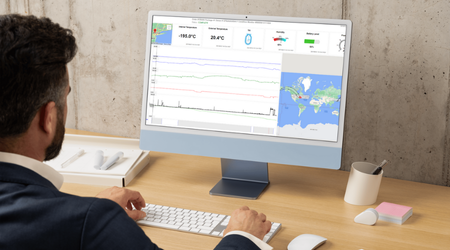
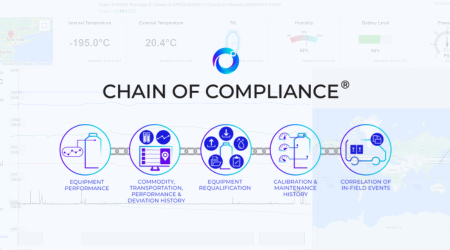
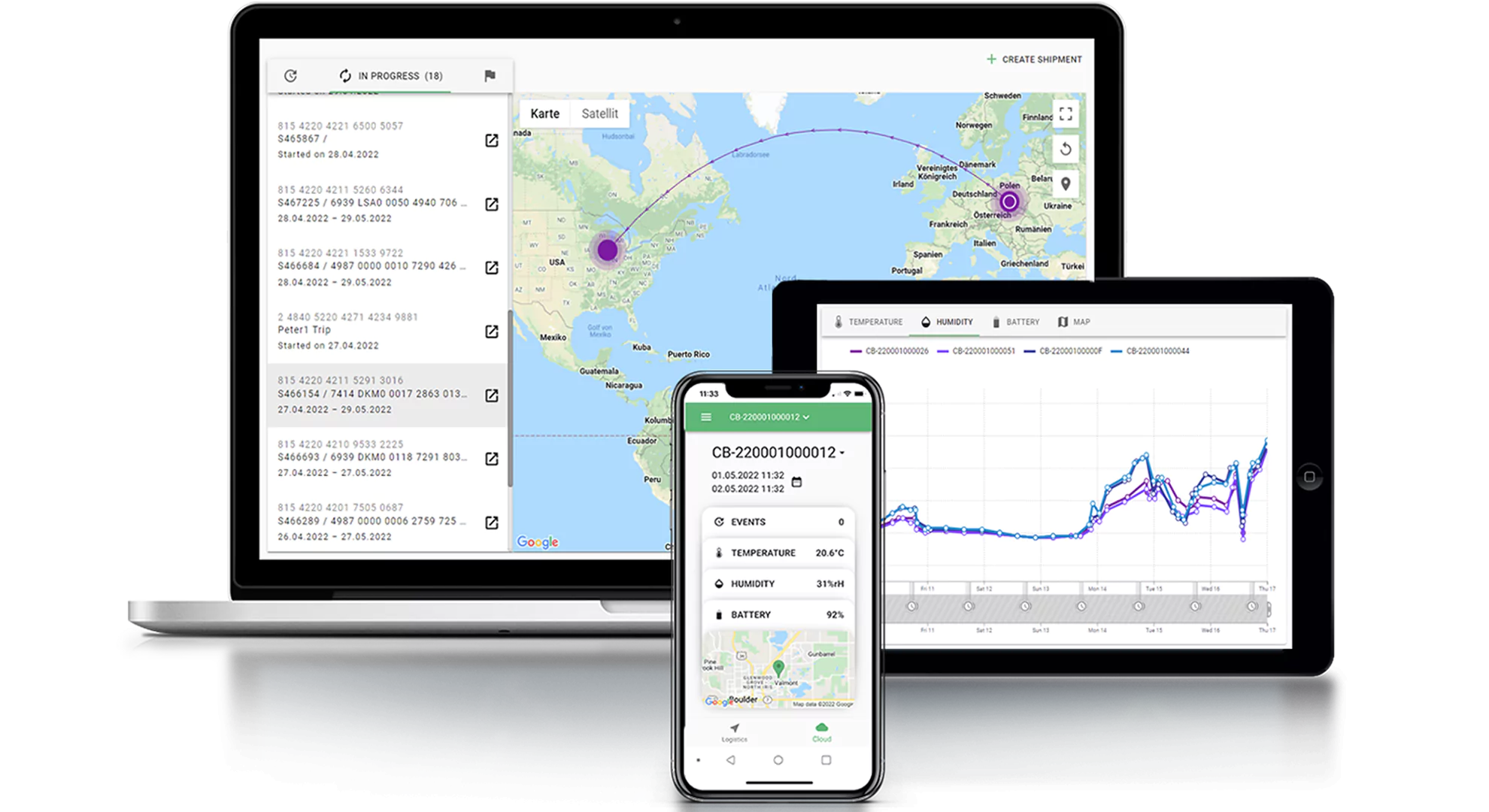
Cryoport Systems is leading the way in innovative solutions that deliver unmatched supply chain control for sensitive biological materials. Our technology ecosystem integrates industry-leading digital platforms with smart sensors and validated, ISO-certified processes to reduce risk at every stage. Our proprietary Cryoportal® logistics management system serves as a central hub for ordering and data history, connecting global operations with comprehensive data and analytics that empower proactive decision making and ensure audit-ready data is at your fingertips when it’s needed. Smartpak™ condition monitoring devices are integrated into all of our custom-engineered shipping systems, tracking critical parameters like temperature, location, tilt, shock, and humidity throughout transit. These data streams feed into our Chain of Compliance®, creating a verifiable audit trail of all shipment and equipment history that strengthens regulatory compliance and simplifies quality oversight.
Beyond monitoring, we set new standards for safety with our Veri-Clean® decontamination process, the industry’s only independently-verified cleaning protocol, proven to eliminate 99.9999% of contaminants like bacteria, viruses, and fungi, implemented alongside rigorous ISO-certified requalification and quality processes that ensure every shipping system is cleaned and validated before every use. For therapies with unique needs, our custom packaging and accessory development delivers tailored solutions for innovative therapeutics. Together, these innovations form a connected ecosystem that transforms complexity into confidence, helping life science organizations move faster and scale smarter.
At Cryoport Systems, quality is engineered into every process. From ISO 21973 certification to Chain of Compliance® traceability and Veri-Clean® validated decontamination, we set the standard.
- Cryoportal®
Centralized visibility and control for global supply chains
Learn More >> - Smartpak™ Integrated Condition Monitoring
Continuous monitoring of shipments for temperature, location, and handling data
Learn More >> - Chain of Compliance®
A verifiable audit trail for regulatory confidence
Learn More >> - Veri-Clean®
Validated decontamination of shipping systems
Learn More >> - Tec4med Sensor Technology
Advanced IoT capabilities for proactive risk management
Learn More >> - Custom Packaging & Accessory Development
Tailored solutions for unique therapy requirements
Learn More >>
Technology That Powers Every Market We Serve
From cell and gene therapy to biologics, animal health, and reproductive medicine
Cryoport Systems technology is engineered to meet the rigorous demands of temperature-controlled supply chains across multiple domains. In cell therapy, we enable precision and predictability for autologous and allogeneic workflows where timing and integrity are non-negotiable. Gene therapy programs benefit from our integrated monitoring and compliance framework, safeguarding viral vectors and genetic materials through complex, multi-leg journeys. For biologics, our validated systems and continuous monitoring protect large molecules from degradation while maintaining strict regulatory alignment. Animal health and reproductive medicine programs leverage the same standards of quality and traceability applicable to human therapeutics, ensuring sensitive materials arrive in optimal condition regardless of geography or scale.
Every solution, whether Cryoportal® for centralized visibility, Smartpak™ for condition monitoring, or Chain of Compliance® for audit-ready documentation, works in concert to reduce variability and accelerate progress. By embedding ISO 21973 compliance and GxP best practices into every process, we deliver a platform that transforms logistics into a sophisticated end-to-end supply chain platform approach to excellence in the life sciences.