Cryoportal®
Logistics Management Platform

Cryoportal® is the Digital Backbone of Advanced Supply Chains
Centralized visibility, predictive analytics, and integrated monitoring that is engineered for precision and compliance
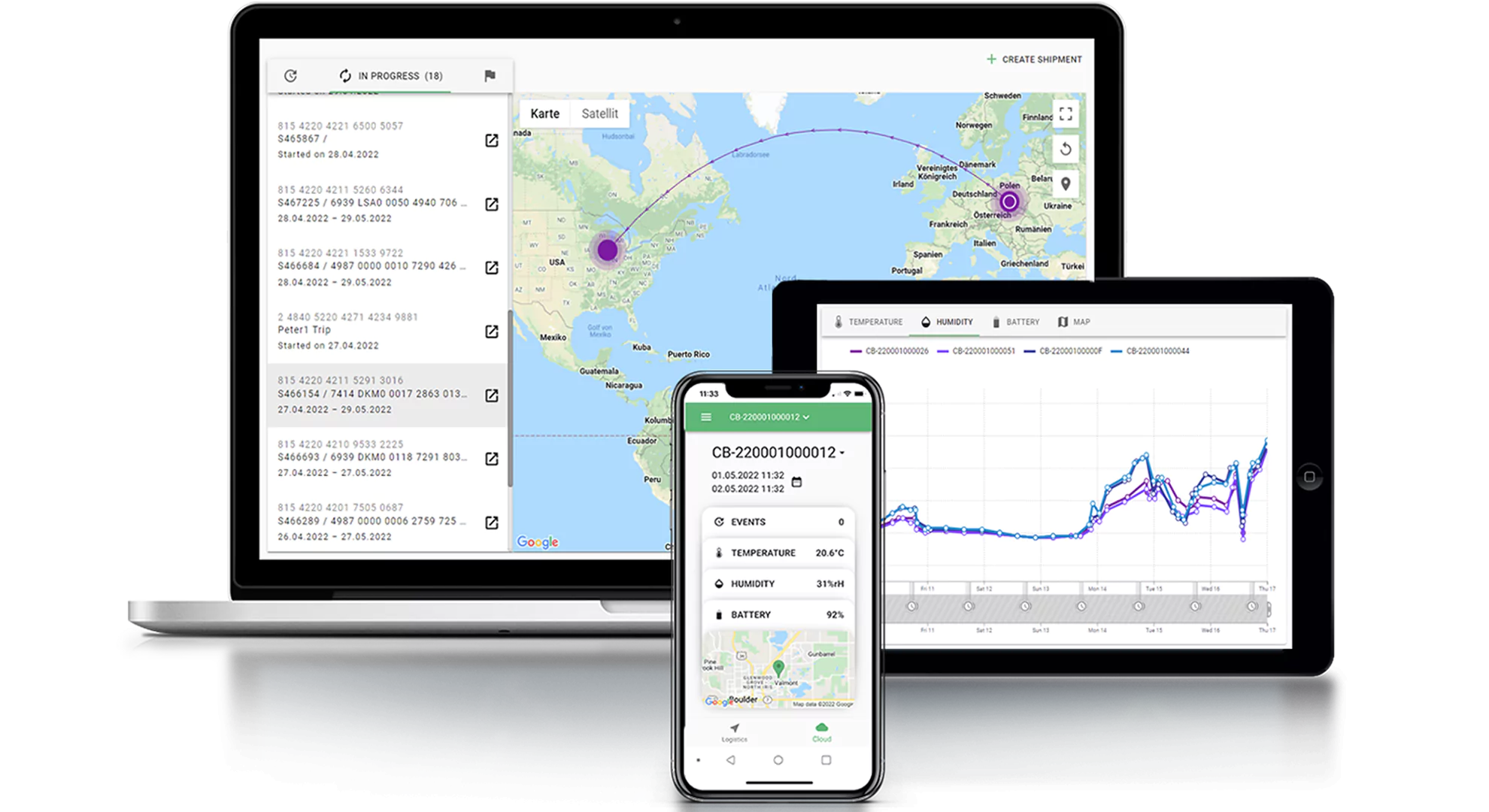
The Cryoportal® logistics management platform is the intelligence layer that powers Cryoport Systems’ global supply chain platform. Designed for cell and gene therapies and supporting additional life sciences applications like biologics, animal health, and reproductive medicine, Cryoportal connects every shipment with every site and stakeholder through a secure, cloud-based environment. It delivers visibility into temperature, location, and other critical parameters of every shipment throughout transit to confirm therapeutic integrity. In a single platform, a shipment’s inventory, ordering, tracking, documentation, and reporting is compiled into a single, user-friendly interface. Automated documentation simplifies audits and regulatory submissions, strengthening compliance with ISO 21973 and GxP standards. Role-based access ensures security while supporting collaboration across global teams.
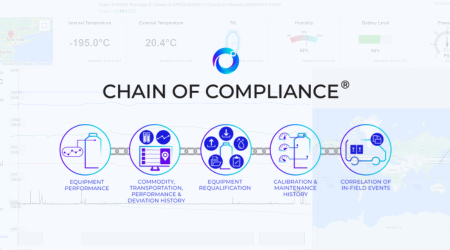
Cryoportal scales seamlessly from early-phase clinical trials to commercial programs, adapting to evolving operational needs. By unifying data streams from Smartpak™ integrated condition monitoring devices embedded in our custom-engineered shipping systems and Chain of Compliance® validated processes and protocols, Cryoportal transforms monitoring into actionable insight, offering a strategic advantage to life science organizations committed to quality and performance.
At Cryoport Systems, quality is engineered into every process. From ISO 21973 certification to Chain of Compliance® traceability and Veri-Clean® validated decontamination, we set the standard.
- Continuous Temperature Control
Smartpak™ tracks temperature throughout transit for uncompromised integrity. - Live View Integration
Continuous data streams into Cryoportal® for visibility and decision-making. - Geolocation Tracking
Monitor shipment location across global routes and customs checkpoints. - Multi-Range Support
Compatible with cryogenic, frozen, refrigerated, and controlled room temperature shipments. - Proactive Alerts
Immediate notifications for excursions, deviations, or handling anomalies. - Exception Management
Embedded workflows for rapid corrective actions and compliance assurance. - Audit-Ready Records
Automated documentation simplifies regulatory submissions and inspections.
Smartpak™ Continuous Monitoring
With Live View® technology delivering shipment intelligence for proactive risk management
Smartpak™ sensors are the cornerstone of Cryoportal’s Live View® capability, providing continuous monitoring of critical conditions throughout transit. These sensors capture temperature, location, and handling data (like tilt, shock, and humidity) at every stage, ensuring materials remain within validated parameters.
Live View integrates this data into Cryoportal® for instant visibility, enabling our 24/7/365 customer support teams to act before issues escalate. Alerts for deviations trigger rapid corrective actions to protect therapeutic integrity.
Smartpak™ integrated condition monitoring supports multiple temperature ranges, including cryogenic, frozen, refrigerated, and controlled room temperature, making it versatile for diverse applications. Geolocation tracking enhances security and transparency across borders and customs checkpoints. Exception management workflows are embedded to streamline resolution and maintain compliance.
By combining Smartpak™ with Cryoportal®, monitoring becomes predictive rather than reactive. Together, they deliver confidence that every shipment arrives as intended… on time, in range, and fully documented to have remained within expected parameters from origin to destination.
This proprietary and enhanced logistics management system is validated to demonstrate compliance with 21 CFR Part 11 (Code of Federal Regulations) and ISPE GAMP (International Society for Pharmaceutical Engineering, Good Automated Manufacturing Practices).