Supply Chain Archives

04/14/2025
Enabling the Outcome Through Enhancing Patient Accessibility
Cell and Gene Therapy (CGT) has emerged as one of the most transformative areas in modern medicine, shaping the future of healthcare and changing the face of next-generation therapeutic approaches. Yet, despite the continued scientific progress, a critical challenge persists in ensuring these therapies are accessible to the patients who need them most. This challenge was the focus of the keynote address at the Advanced Therapies Congress in London, delivered by Aruna Mor, Chief Commercial Officer at Cryoport Systems, where she spoke about to the state of patient access within the field of advanced therapeutics.
03/21/2025
Optimizing ATMP Cryopreservation: How IntegriCell™ Services in Belgium Ensure Cell Viability and Supply Chain Integrity
Cryopreservation is quickly becoming a critical yet often underestimated component of the Advanced Therapy Medicinal Product (ATMP) supply chain. The success of cell-based therapies depends on maintaining cell viability, stability, and consistency, yet variability in cryopreservation methods has compromised therapeutic outcomes.
03/12/2025
The Gateway to Europe: How Cryoport Systems’ Stevenage Supply Chain Hub Supports ATMP Development
For UK-based Advanced Therapy Medicinal Product (ATMP) developers, access to European clinical trial sites and commercial markets is a critical component of a successful growth strategy. Yet, navigating regulatory barriers, customs challenges, and supply chain complexities can create unnecessary delays, increase costs, and put the integrity of sensitive biologics at risk.
02/04/2025
Advancing Patient Outcomes Through Integrated Supply Chain Solutions
The landscape of advanced therapies is consistently shifting, requiring a seamless, high-quality supply chain to ensure the safe and effective delivery of life-saving treatments. In a recent interview at Advanced Therapies Week, Cryoport Systems leaders shared insights into how their integrated approach is shaping the future of supply chain management for the biopharmaceutical industry.
01/06/2025
The Future of Gene Therapy Logistics – A Pivotal Moment
Gene therapy is set to transform healthcare, but its success depends on logistics systems that can adapt and scale in line with the industry’s growth. Cryoport Systems is proud to support this exciting frontier with logistics solutions that prioritize product integrity, security, and compliance.
12/30/2024
Supporting Compliance and Reducing Risk in Gene Therapy Logistics
When it comes to high stakes logistics in the gene therapy sector, adherence to regulatory standards isn’t just a box to check—it’s essential to delivering therapies that maintain integrity from production to patient. Cryoport Systems understands the unique risks in this space and has built compliance and risk mitigation into the very design of the Cryoport Elite™ Ultra Cold shipping system.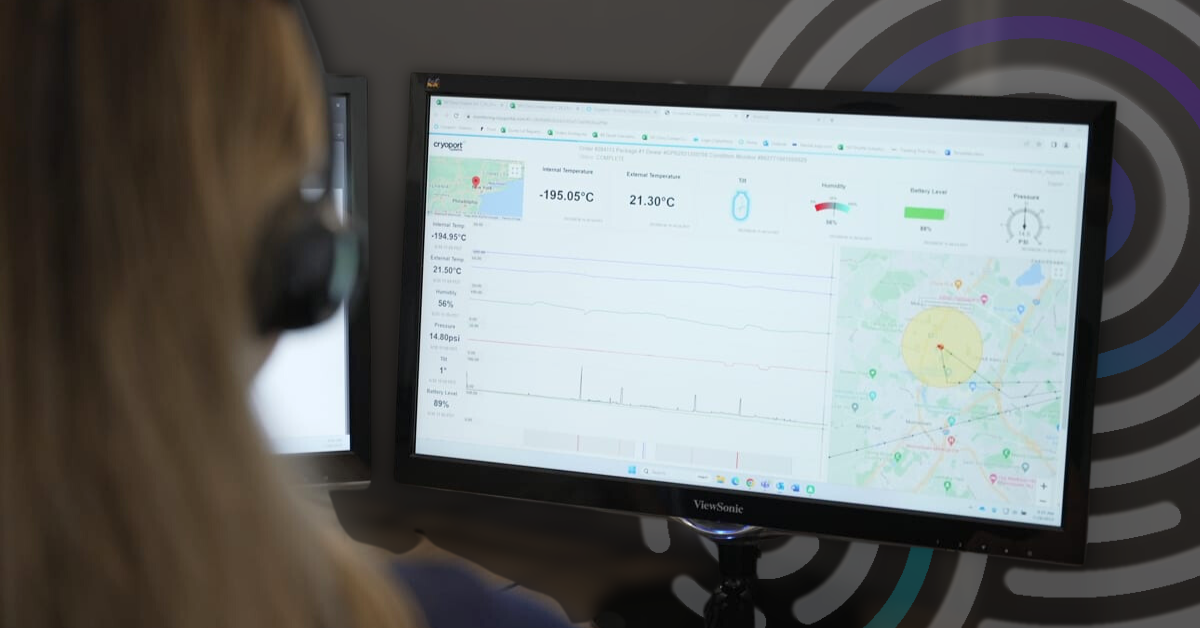
12/16/2024
Solving Key Gene Therapy Logistics Challenges with Advanced Technology
Cryoport Systems developed the Cryoport Elite™ Ultra Cold shipping system with one mission in mind: to ensure that gene therapies reach their destination intact, uncompromised, and ready for patient treatment.
06/25/2024
The Cryoport Elite® Shipping System with Mike Dybicz, Cryoport Systems’ SVP & CPDO
In 2023, Cryoport Systems announced the launch of the Cryoport Elite™ shipping system.
05/20/2024
Cryoport Elite® Shipping System: Industry-leading Advanced Therapy Support
The life sciences industry requires a robust shipping system that can handle the risks associated with transporting high-value commodities such as advanced therapies.Categories
- All (57)
- Industry Insights (16)
- Managing the Cold Chain (16)
- Navigating Logistics (21)
- Patient Access and Awareness (4)
